Abstract
Background: A number of studies have reported elevated incidence of 25-OH-vitamin D deficiency among patients with multiple myeloma (MM). Several studies have found association between vitamin D levels and factors associated with survival, including ISS stage at diagnosis. However, the impact of vitamin D deficiency on MM prognosis is not entirely clear. Also, in general, both the incidence and the impact of vitamin D deficiency differ substantially by race. Here, we investigate the impact of vitamin D deficiency on prognosis in a large and racially heterogenous patient population with MM in the Veterans Affairs (VA) system.
Methods: We used the VA's nationwide Corporate Data Warehouse to identify patients diagnosed with symptomatic MM from 1999 to 2017. Various demographic and laboratory data was collected including age, race, 25-OH-vitamin D levels, and ISS stage at diagnosis as well as survival outcome data. Details of therapies received was also available which indicted similar access to all newer agents approved for myeloma for both African American (AA) and Caucasian patients.
Results: We identified 15,717 patients diagnosed with MM (3353 AA and 9070 Caucasian), of whom 6675 had vitamin D measurements within 2 months of diagnosis (1959 AA and 4398 Caucasian). Median serum vitamin D levels were significantly lower among AA patients (21.8 ng/mL) than Caucasians (28.6 ng/mL; p<0.0001). No difference in median vitamin D levels was observed across ISS stage at diagnosis (p=0.7575), but a significant positive correlation (ρ=0.166; p<0.0001) was found between vitamin D levels and age at diagnosis.
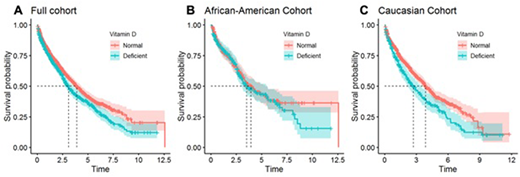
We evaluated the ability of serum vitamin D level to predict overall survival (OS) in patients with MM using a cut-off of 20ng/mL. Patients with vitamin D deficiency (<20ng/mL) had a significantly worse prognosis than patients with normal levels (≥20ng/mL) (Fig 1A). Specifically, median OS was 3.10 years (95% CI 2.73-3.52) for patients with vitamin D deficiency, compared to 3.91 years (95% CI 3.59-4.38) for patients with normal serum vitamin D. Univariate Cox proportional hazard analysis also showed that vitamin D deficiency is a significant predictor of OS after MM diagnosis (HR 1.24; P=0.0021), and vitamin D deficiency remained an independent predictor of OS under multivariate analysis in which adjustments were made for race, age, and stage at diagnosis (HR 1.28; P=0.0385).
The analyses were repeated for AA and Caucasian patients separately. Among AA patients, serum vitamin D was not a significant predictor of OS in univariate (P=0.5096) or multivariate analysis (P=0.6923), while it was still a strong predictor among Caucasian patients in both univariate (HR 1.38; P=0.0006) and multivariate analysis (HR 1.45; P=0.0048). Median OS is 3.54 years (95% CI 2.99-5.52; n=255) for AA patients with vitamin D deficiency and 3.95 years (3.25-5.35; n=296) with normal levels. Among Caucasians, median OS is 2.71 years (2.18-3.47; n=273) for deficient and 3.87 years (3.59-4.42; n=885) for normal. Kaplan-Meier plots (Fig. 1B and 1C) illustrate the observed OS curves for the two subgroups. Since levels of vitamin D were lower in AA patients, a lower cut-off of 10 ng/mL was also tested. Even using this lower cutoff, vitamin D deficiency was not a statistically significant predictor of OS in univariate (HR 1.33; P=0.0781) or multivariate analysis (HR 1.09; P=0.7039), though the number of AA patients with vitamin D <10 ng/ML is small (n=73).
Conclusions: Vitamin D deficiency is a significant predictor of survival among patients diagnosed with MM, even after accounting for race, age, and ISS stage. However, this relationship is only
observed in Caucasian patients and not observed among AA patients. Studies are ongoing to evaluate impact of Vitamin D deficiency of disease presentation including bone disease as well as genetic characteristics. This investigation highlights the need to assess the underlying biological mechanism responsible for the observed impact of vitamin D deficiency across race in MM.
Yellapragada:Novartis: Employment; Celgene: Research Funding; Takeda: Research Funding. Munshi:OncoPep: Other: Board of director.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal